2.4 Personal Protective Equipment (PPE) and Respiratory Protective Equipment (RPE)
2.4.1 Surgical masks
A type IIR fluid resistant surgical mask should be worn when caring for a patient with a suspected/confirmed infectious agent spread by the droplet route.
Surgical masks worn by patients with suspected/confirmed infectious agents spread by the droplet or airborne routes, as a form of source control, should meet type II or IIR standards.
During periods of increasing or high prevalence of transmissible respiratory infection within healthcare areas, for example emergency departments or throughout a hospital facility, local health boards may undertake a risk assessment and advise wider use of fluid resistant type IIR surgical face masks by healthcare workers in these areas as part of a suite of enhanced control measures. Local epidemiology should be used to inform the risk assessment.
2.4.2 Eye/face protection
Eye and face protection should be worn in combination with:
- a fluid resistant type IIR surgical mask when caring for symptomatic patients infected with droplet transmitted infectious agents
- a fluid resistant FFP3 respirator when caring for symptomatic patients infected with an airborne transmitted infectious agent
Eye and face protection should be worn:
- when there is an anticipated risk of splashing and/or spraying of blood or bodily fluids, and
- when caring for patients with novel infectious agents including pandemic influenza
2.4.3 Aprons/Gowns
The type of aprons or gowns used in health and care settings should be selected based on the task being undertaken, and the anticipated levels of body fluid exposure.
A disposable apron should be worn when in contact with a patient’s environment or when providing direct care to those with known or suspected infection or known or suspected to be colonised/infected with a transmissible infectious agent.
A fluid repellent gown should be used if excessive splashing or spraying is anticipated.
A full body fluid repellent gown should be worn when conducting AGPs on patients known or suspected to be infected with a respiratory infectious agent.
Resources
Further information can be found in the Aprons/Gowns literature review.
2.4.4 Gloves
Gloves must:
- be worn when exposure to blood, body fluids, (including but not limited to secretions and/or excretions), non-intact skin, lesions and/or vesicles, mucous membranes, hazardous drugs and chemicals, e.g. cleaning agents is anticipated/likely
- Gloves are a single-use item and should be donned immediately prior to exposure risk and should be changed immediately after each use or upon completion of a task
- never be worn inappropriately in situations such as to go between patients, move around a care area, work at IT workstations
- be changed if a perforation or puncture is suspected or identified
- be appropriate for use, fit for purpose and well-fitting
- not be worn as a substitute to hand hygiene
Double gloving is only recommended during some Exposure Prone Procedures (EPPs), for example orthopaedic and gynaecological operations, or when attending major trauma incidents and when caring for a patient with a suspected or known High Consequence Infectious disease. Double gloving is not necessary at any other time.
Resources
For appropriate glove use and selection see Appendix 5.
Further information can be found in the Gloves literature review.
2.4.5 RPE
PPE must still be used in accordance with SICPs when using Respiratory Protective Equipment. See Chapter 1.4 for PPE use for SICPs.
Where it is not reasonably practicable to prevent exposure to a substance hazardous to health (as may be the case where healthcare workers are caring for patients with suspected or known airborne micro-organisms) the hazard must be adequately controlled by applying protection measures appropriate to the activity and consistent with the assessment of risk. If the hazard is unknown the clinical judgement and expertise of IPC/HP staff is crucial and the precautionary principle should apply.
Respiratory Protective Equipment (RPE), for instance FFP3 and facial protection, must be considered when:
- a patient is admitted with a known/suspected infectious agent/disease spread wholly by the airborne route
- carrying out aerosol generating procedures (AGPs) on patients with a known/suspected infectious agent spread wholly or partly by the airborne or droplet route
See Appendix 16 for the extant list of Aerosol Generating Procedures which require the application of airborne precautions and details of associated Post AGP Fallow times.
Filter Face Piece 3 (FFP3) Respirators
Where staff have concerns, they may choose to wear an FFP3 respirator rather than a fluid-resistant surgical mask (FRSM) when providing patient care, provided they are fit tested. This is a personal PPE risk assessment.
All tight fitting RPE (for instance FFP3) respirators must be:
- fit tested (by a competent fit test operator) on all healthcare staff who may be required to wear a respirator to ensure an adequate seal/fit according to the manufacturers’ guidance
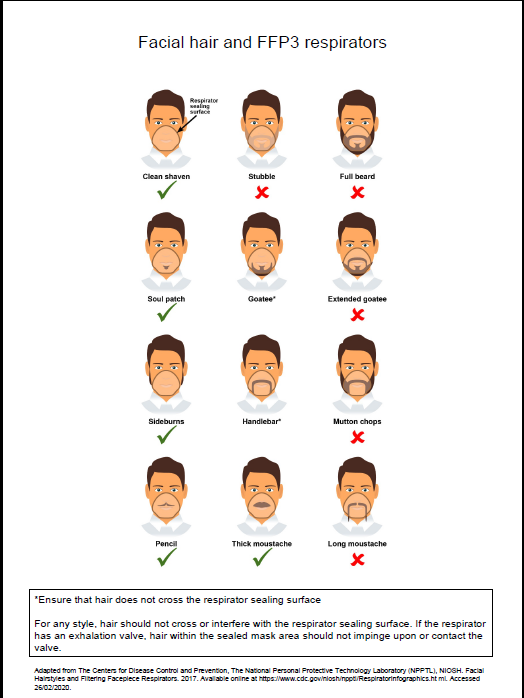
- fit checked (according to the manufacturers’ guidance) every time a respirator is donned to ensure an adequate seal has been achieved. The poster below gives further information on compatibility of facial hair and FFP3 respirators and can be used when fit testing and fit checking
- single use (disposable) and fluid-resistant. Valved respirators may be shrouded or unshrouded. Respirators with unshrouded valves are not considered to be fluid-resistant and therefore should be worn with a full face shield if blood or body fluid splashing is anticipated
- non valved if a sterile procedure is being performed at the same time as an AGP requiring a respirator to be worn. An MHRA safety alert can be viewed.
- compatible with other facial protection used, for instance protective eyewear, so that this does not interfere with the seal of the respiratory protection. Prescription eyeglasses and contact lenses should not be considered a form of eye/face protection. If wearing a valved, non-shrouded FFP3 respirator a full face shield/visor must be worn
- always put on before entry into the patient room/area and prior to performing an aerosol generating procedure (AGP) and removed in an anteroom/lobby or in a safe area, for example outside the isolation/cohort room/area. All other PPE should be removed in the patient care area
- changed after each use. Other indications that a change in respirator is required include: if breathing becomes difficult, if the respirator becomes wet or moist, damaged or obviously contaminated with body fluids such as respiratory secretions.
Resources
Poster on compatibility of facial hair and FFP3 respirators can be used when fit testing and fit checking.
Further information regarding fitting and fit checking of respirators can be found on the Health and Safety Executive website.
National Priority Risk Categorisation for face fit testing with FFP3
The following risk categorisation is the minimum requirement for staff groups that require FFP3 fit testing. NHS boards can add to this for example where high-risk units are present. This categorisation is inclusive of out of hours services.
Level 1 – Preparedness for business as usual
Staff in clinical areas most likely to provide care to patients who present at healthcare facilities with an infectious pathogen spread by the airborne route; and/or undertake aerosol generating procedures. These are A&E, ICU, paediatrics, respiratory, infectious diseases, anaesthesia, theatres, Chest physiotherapists, Special Operations Response Team (Ambulance), A&E Ambulance Staff, Bronchoscopy Staff, Resuscitation teams, mortuary staff.
Level 2 – Preparedness in the event of emerging threat
Staff in clinical setting likely to provide care to patients admitted to hospital in the event of an emerging threat, for example Medical receiving, Surgical, Midwifery and Speciality wards, all other ambulance transport staff.
In the event of an ‘Epidemic/Pandemic’ Local Board Assessment as per their preparedness plans will apply.
The decision to wear an FFP3 respirator/hood should be based on clinical risk assessment, for example task being undertaken, the presenting symptoms, the infectious state of the patient, risk of acquisition and the availability of treatment.
Resources
For a list of organisms spread wholly or partly by the airborne (aerosol) or droplet routes see Appendix 11.
Further information can be found in the aerosol generating procedures literature review.
Powered respirator hoods
Powered respirator hoods are an alternative to FFP3 respirators for example when fit testing cannot be achieved.
Powered hoods must be:
- single use (disposable) and fluid resistant
- the filter must be enclosed with the exterior and the belt able to withstand disinfection with 10,000ppm av.cl.
FFP3 respirator or powered respirator hood
- may be considered for use by visitors if there has been no previous exposure to the infected person or infectious agent; but
- must never be worn by an infectious patient(s) due to the nature of the respirator filtration of incoming air not expelled air.
Work is currently underway by the UK Re-useable Decontamination Group examining the suitability of respirators for decontamination. This literature review will be updated to incorporate recommendations from this group when available. In the interim, ARHAI Scotland are unable to provide assurances on the efficacy of respirator decontamination methods and the use of re-useable respirators is not recommended.
Further information can be found in the Respiratory Protective Equipment (RPE) literature review and the Personal Protective Equipment (PPE) for High Consequence Infectious Diseases (HCIDs) Literature review.
PPE for visitors
PPE may be offered to visitors to protect them from a transmissible infection.
If a visitor declines to wear PPE when it is offered then this should be respected. PPE use by visitors cannot be enforced and there is no expectation that staff monitor PPE use amongst visitors. However, if staff or visitors identify the need for PPE, advice on its correct use should be provided by staff.
When visiting a patient with a known or suspected infection, visitors do not routinely require PPE unless they are providing direct care to the individual they are visiting.
The table below shows the PPE which should be worn where appropriate and when the visitor chooses to do so.
IPC Precaution |
Gloves |
Apron1 |
Face covering/
|
Eye/Face Protection |
|---|---|---|---|---|
|
Transmission Based Precautions (TBPs) |
Not required unless providing direct care which may expose the visitor to blood and/or body fluids i.e. toileting. |
Not required unless providing care resulting in direct contact with the service user, their environment or blood and/or body fluid exposure i.e. toileting, bed bath. |
Where splash/spray to nose/mouth is anticipated during direct care |
Where splash/spray to nose/mouth is anticipated during direct care and/or if within 2 metres of service user with suspected or known respiratory infection |
¹A gown may be selected where excessive splashing or spraying may be anticipated.